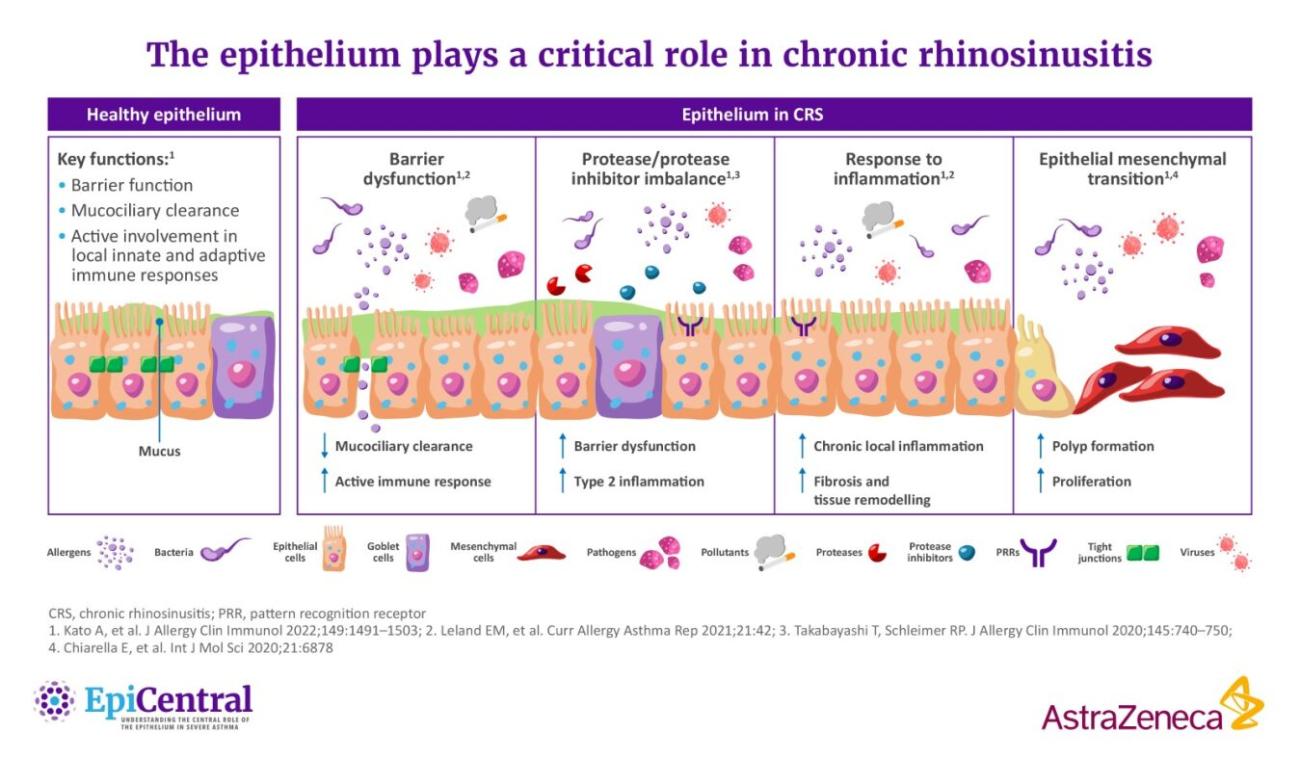
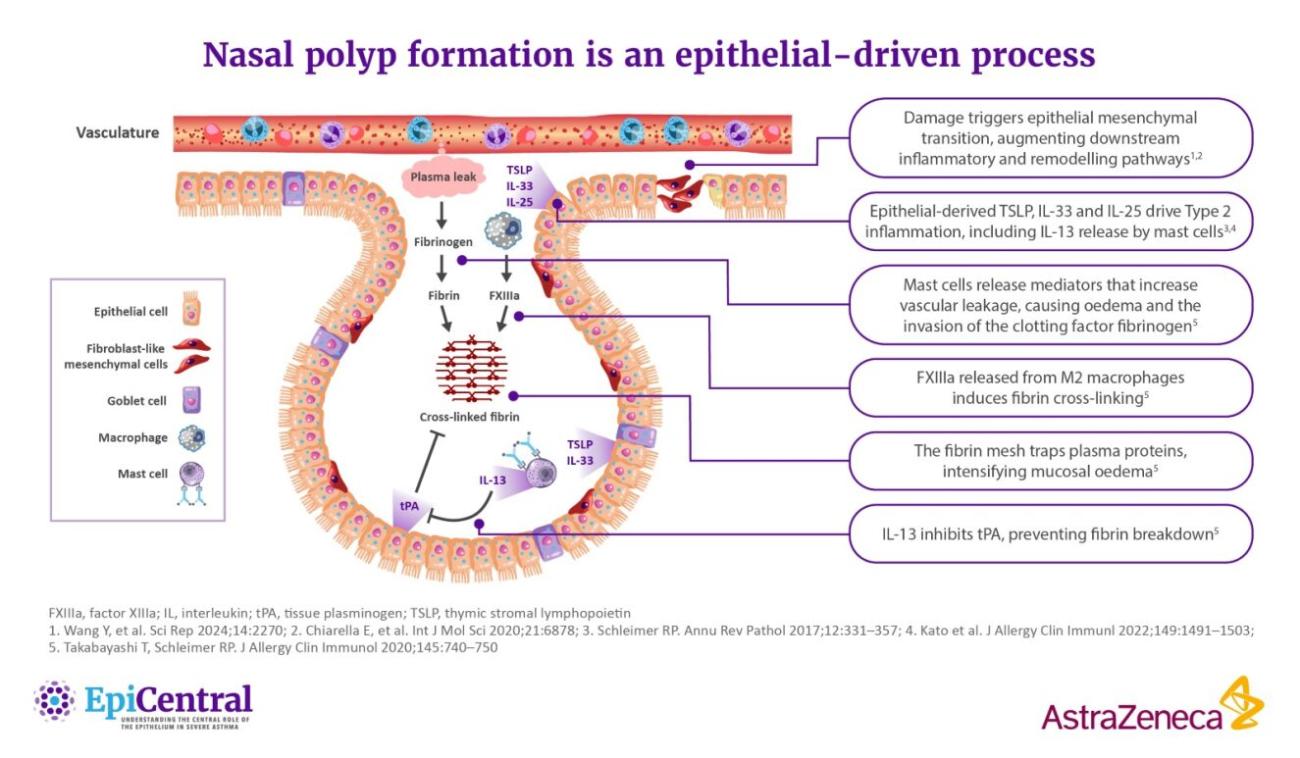
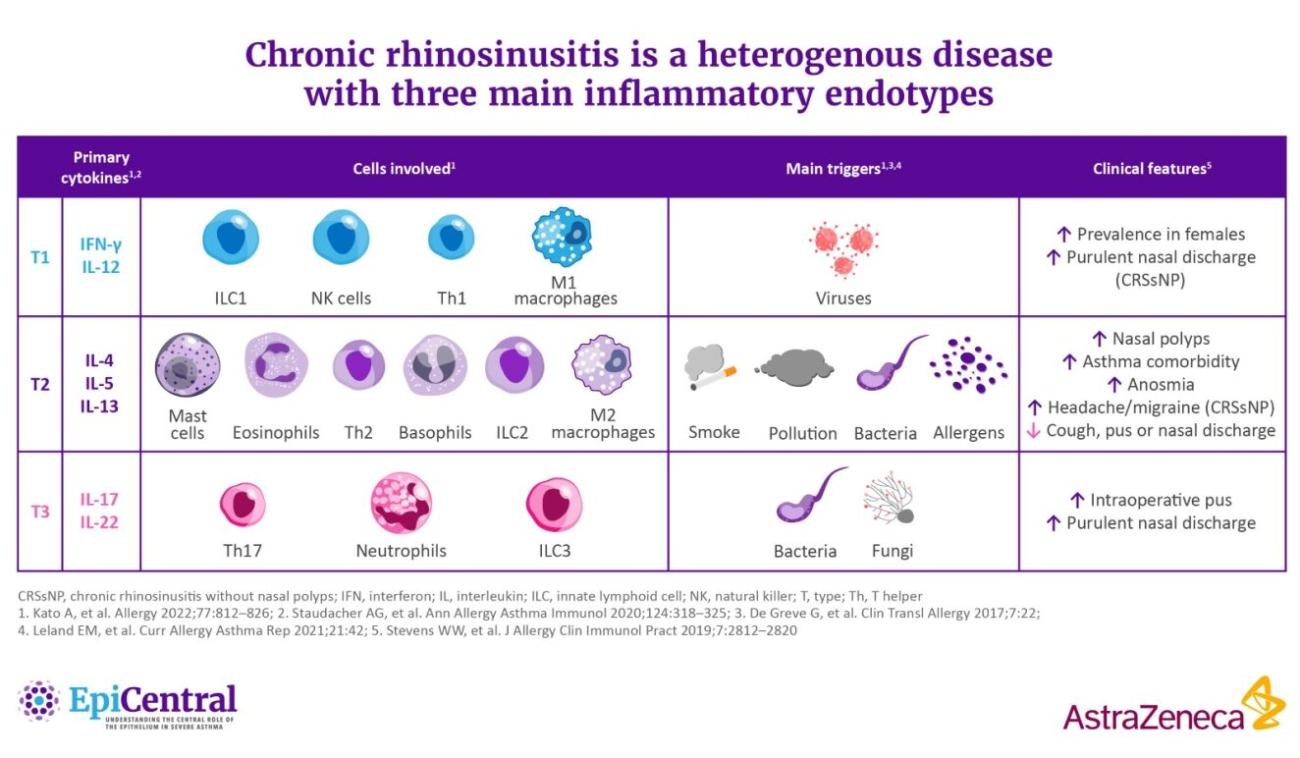
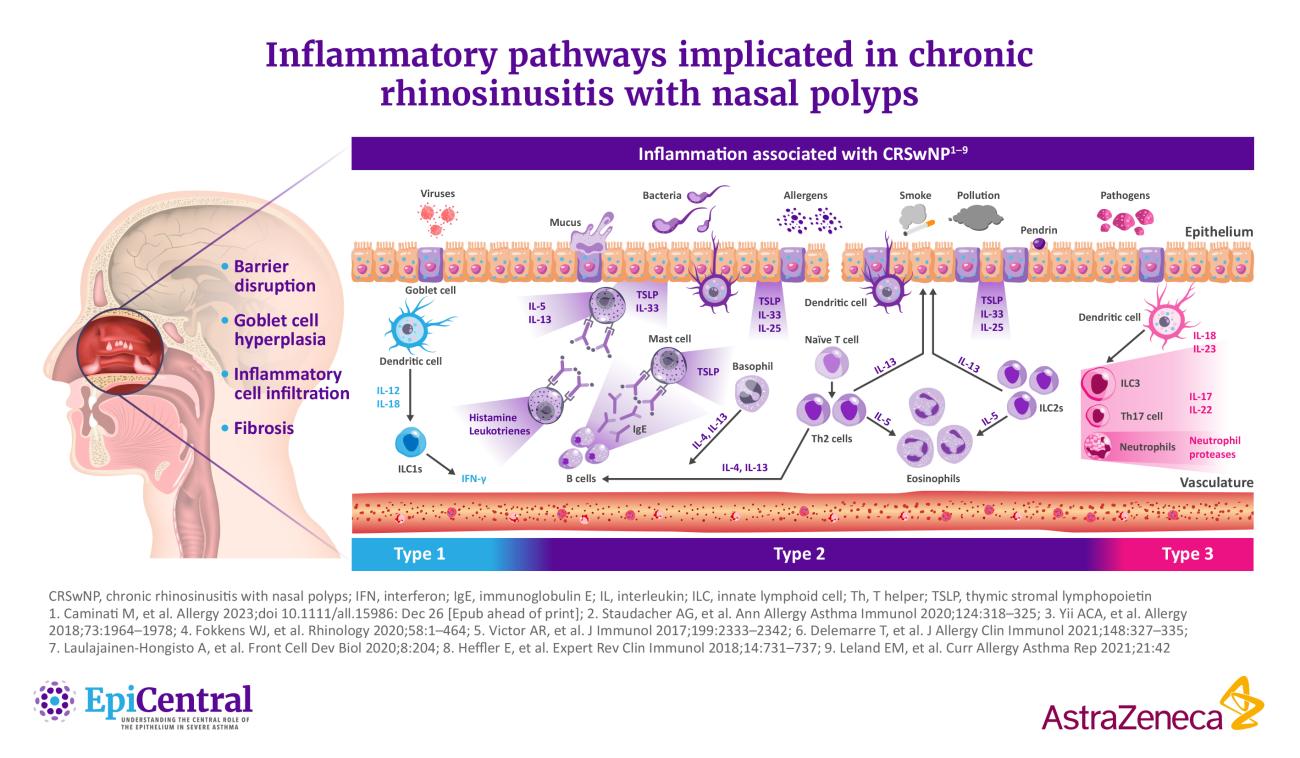
References
1. Wynne M, et al. Am J Rhinol Allergy 2019;33:782–790; 2. Orlandi RR, et al. Int Forum Allergy Rhinol 2021;11:213–739; 3. Sedaghat AR, et al. J Allergy Clin Immunol Pract 2022;10:1395–1403; 4. Claeys N, et al. Front Allergy 2021;2:761388; 5. Zhang M, et al. Int Immunopharmacol 2023;121:110559; 6. Liao B, et al. Allergy 2015;70:1169–1180; 7. Cho SH, et al. J Allergy Clin Immunol Pract 2020;8:1505–1511; 8. Fokkens WJ, et al. Rhinology 2020;58(Suppl. S29):1–464; 9. Benjamin MR, et al. J Allergy Clin Immunol Pract 2019;7:1010–1016; 10. Soler ZM, et al. Laryngoscope 2011;121:2672–2678; 11. Schneider S, et al. J Clin Med 2020;9:925; 12. Kim J-Y, et al. JAMA Otolaryngol Head Neck Surg 2019;145:313–319; 13. Tomoum MO, et al. Int Forum Allergy Rhinol 2015;5:674–681; 14. Hopkins C, et al. Rhinology 2015;53:18–24; 15. Hopkins C, et al. Rhinology 2015;53:10–17; 16. Vennik J, et al. BMJ Open 2019;9:e022644; 17. Hellings PW, et al. Rhinology 2023;61:85–89; 18. De Corso E, et al. J Pers Med 2022;12:897; 19. Price DB, et al. J Asthma Allergy 2018;11:193–204; 20. Fokkens WJ, et al. Allergy 2019;74:2312–2319; 21. van der Veen J, et al. Allergy 2017;72:282–290; 22. DeConde AS, et al. Laryngoscope 2017;127:550–555; 23. Yan B, et al. J Allergy Clin Immunol 2024;doi 10.1016/j.jaci.2024.01.015: Jan 29 [Epub ahead of print]; 24. Vroling AB, et al. Allergy 2008;63:1110–1123; 25. Kato A, et al. J Allergy Clin Immunol 2022;149:1491–1503; 26. Ha J-G, Cho H-J. Int J Mol Sci 2023;24:14229; 27. Harkema JR, et al. Toxicol Pathol 2006;34:252–269; 28. Tu Y, et al. Inflammation 2021;44:1937–1948; 29. Gudis D, et al. Am J Rhinol Allergy 2012;26:1–6; 30. Yan X, et al. Laryngoscope Investig Otolaryngol 2020;5:992–1002; 31. Mullol J, et al. J Allergy Clin Immunol Pract 2022;10:1434–1453; 32. Wang Y, et al. Sci Rep 2024;14:2270; 33. Gong X, et al. Front Immunol 2023;14:1238673; 34. Chan Y, et al. J Otolaryngol Head Neck Surg 2023;52:50; 35. Staudacher AG, et al. Ann Allergy Asthma Immunol 2020;124:318–325; 36. Stevens WW, et al. J Allergy Clin Immunol Pract 2019;7:2812–2820; 37. Zhang Y, et al. J Allergy Clin Immunol 2017;140:1230–1239; 38. Ryu G, et al. Precis Future Med 2022;6:170–176; 39. Schleimer RP. Annu Rev Pathol 2017;12:331–357; 40. Laidlaw TM, et al. J Allergy Clin Immunol Pract 2021;9:1133–1141; 41. Gauvreau GM, et al. Allergy 2023;78:402–417; 42. Duchesne M, et al. Front Immunol 2022;13:975914; 43. Cho SH, et al. J Allergy Clin Immunol Pract 2016;4:575–582; 44. Fokkens W, Reitsma S. Otolaryngol Clin North Am 2023;56:1–10; 45. Lin DC, et al. Am J Rhinol Allergy 2011;25:205–208; 46. Denlinger LC, et al. Am J Respir Crit Care Med 2017;195:302–313; 47. Ek A, et al. Allergy 2013;68:1314–1321; 48. Khan A, et al. Rhinology 2019;57:32–42.